

Akanksha Varanasi
Monta Vista High SchoolClass of 2023Cupertino, California
About
My name is Akanksha, and I am a high school student interested in science and technology, specifically biology and engineering. For the past year, I've been researching genetics (specifically the CRISPR/Cas9 gene-editing system) for my Polygence projects, and I'm excited to share my work!Projects
- "The Applications of the CRISPR/Cas9 Gene-Editing System in Treating Human Diseases" with mentor Ellie (June 8, 2022)
Project Portfolio
The Applications of the CRISPR/Cas9 Gene-Editing System in Treating Human Diseases
Started Nov. 1, 2021
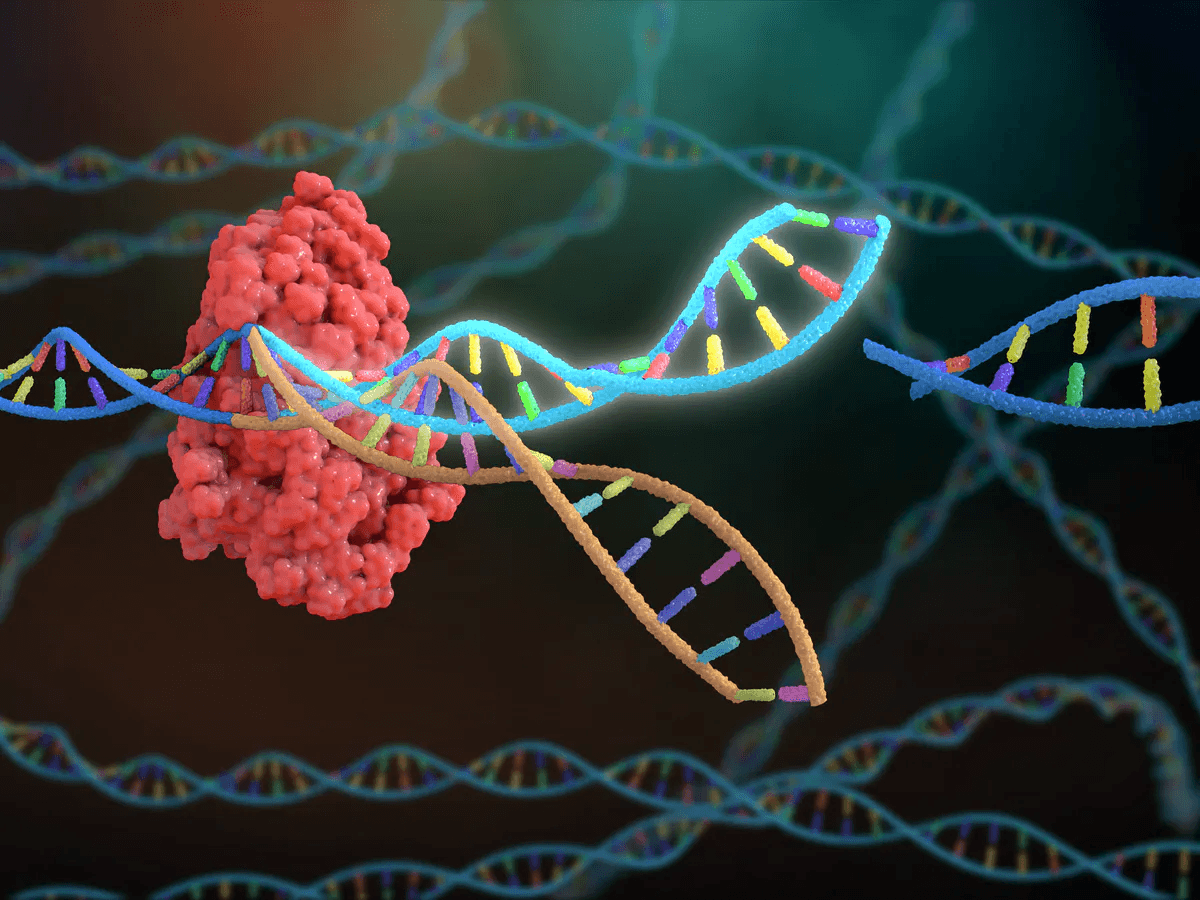
Abstract or project description
In the early 2010s, scientists realized that CRISPR/Cas9, a bacterial immune defense system against viruses that involves the CRISPR-associated protein #9 (Cas9) endonuclease enzyme, single-guide RNAs (sgRNAs), and PAM recognition, could be used in the context of gene editing to intentionally manipulate genes, essentially changing gene expression and regulation in such a way that would allow for a customized genome. Since then, CRISPR technology has revolutionized medical research and the biotechnology industry, and its newfound capabilities have scientists asking if CRISPR can be used to modify genes in such a way that would cure or treat certain harmful or life-threatening diseases. There have been CRISPR-based clinical studies done to treat β-thalassemia (TDT), sickle-cell disease (SCD), the human immunodeficiency virus (HIV), and several other genetic and non-hereditary diseases, but there is still a long way to go before CRISPR can become a widespread treatment for many more such diseases (Ebina et al., 2013; Esrick et al., 2021; Frangoul et al., 2021). Currently, researchers are looking to see if CRISPR is an accurate, specific, non-harmful, and effective treatment for these diseases, which means addressing and eliminating potential concerns about its safety and efficacy through extensive pre-clinical and clinical research, as well as overcoming moral and social obstacles. In this review, I will look at how the CRISPR/Cas9 gene-editing system can be applied in humans to prevent, cure, or treat these diseases, as well as what needs to be done before the CRISPR/Cas9 system can be made publicly available as a medical treatment for diseases.
Project Portfolio
Using CRISPR/Cas9 Technology to Genetically Transform and Edit the rpsL Amino Acid Sequence of E. Coli Bacteria to Allow it to Grow on Streptomycin Media
Started Apr. 29, 2022
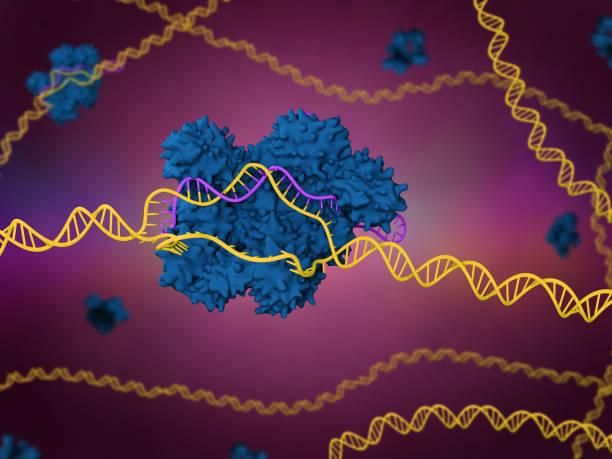
Abstract or project description
The CRISPR/Cas9 system is a naturally-derived gene-editing system that originates from bacterial immune systems. Its main components are the CRISPR-associated protein #9 (Cas9) endonuclease enzyme, a single-guide RNA (sgRNA) made up of CRISPR RNA (crRNA) and tracrRNA, and a protospacer adjacent motif (PAM) sequence (Jinek et al., 2012). Cas9 complexes with the sgRNA to create a ribonuclear protein that binds to template DNA complementary to the target DNA sequence and, following PAM recognition, cuts the DNA at the intended target site. The DNA is repaired with respect to the template DNA, which usually contains a purposeful mutation or base change to achieve the expected result—often a genetic change that expresses a different phenotype or changes the function of the gene.
In a three-day lab experiment—including a preparation day before and a results-measuring/clean-up day after—I successfully used the CRISPR/Cas9 gene-editing system to genetically modify the 43rd amino acid, Lysine (K), in the ribosomal subunit protein (rpsL) amino acid sequence of non-pathogenic DH5ɑ Escherichia coli bacteria, and change it to a Threonine (T). This K43T genetic mutation allowed the bacteria to survive on Luria-Bertani (LB) media containing the antibiotic streptomycin, which normally prevents bacteria from growing because it binds to the ribosomes of the bacteria and inhibits its ability to make proteins. However, the genetic mutation induced by CRISPR stops streptomycin from binding to the bacterial ribosomes, thus allowing the bacteria to grow on the antibiotic-containing LB plate.